On July 24th, 2023, the Journal of Thrombosis and Haemostasis preprinted an article [1] by Connors and Ariëns entitled “Uncertainties about the Roles of Anticoagulation and Microclots in Post-acute Sequelae of SARS-CoV-2 Infection”. While it is not entirely clear what were either the motivation or purposes of that article (referred to as a ‘concept’ under ‘author contributions’), its main thrust seems to be to wish to attack the nature, existence, and the importance of fibrin amyloid microclots in PASC/Long COVID (and other diseases). It does so by including a miasma of misstatements, inuendoes, selective and missing citations, and a seemingly purposeful misunderstanding or ignorance of much else that has been published that presumably exacerbates their ‘uncertainties’ but does no credit to either the authors or the journal.
Eventually a much diluted/bowdlerised version of what was sent in by us as a rebuttal was (no doubt grudgingly) published by the journal [2], with most of the relevant references cut, but since the opponents of our work seem to think it is open season to continue to attack us with seemingly no obvious comeback it is time to give full chapter and verse of precisely how poor that piece was in terms of its analysis of what the papers they cite actually said. Other rebuttals will also be posted on this site, since my experience shows that if you keep calling out bad science their proponents eventually stop the unscientific attacks.
The purpose of this blog is to set the record straight in an Open Access manner by pointing out the full (or at least a considerably fuller) set of the pertinent facts, along with all the references and some more recent developments.
First set of mis-citations and non-citations
The first gambit states of anticoagulation that there is “no demonstrated benefit in most hospitalized populations, and no need for anticoagulation in ambulatory and post-discharge patients. (2-13)” [1] (the mis-punctuation is left as it is throughput, as we quote directly). The next paragraphs analyse what those citations 2-13 of [1] actually found.
Their reference 2 [3] is a study of anticoagulation in critically ill patients hospitalised in Intensive Care Units, by which time it was mostly too late for anticoagulation (or indeed anything else) to save them. By contrast, the exactly companion paper on non-critically ill patients, by essentially the same authors [4], is reference 3 of Connors and Ariëns; far from showing ‘no demonstrated benefit’ it actually concludes “In noncritically ill patients with Covid-19, an initial strategy of therapeutic-dose anticoagulation with heparin increased the probability of survival to hospital discharge {our boldface} with reduced use of cardiovascular or respiratory organ support as compared with usual-care thromboprophylaxis.” It is hard to imagine a more inaccurate style of reporting than that of [1].
Reference 4 [5] involved a comparison of intermediate-vs-standard anticoagulation of patients in ICUs, and found no difference, but the controls also received anticoagulation, so this is hardly a reflection of “no need for anticoagulation”.
Reference 5 of [1] is another comparison of ‘therapeutic’ vs ‘prophylactic’ doses of heparin [6] (so the ‘controls’ also received anticoagulation). This said “Deaths occurred in four patients (1.8%) assigned to therapeutic heparin and 18 patients (7.6%) assigned to prophylactic heparin (0.22, 0.07 to 0.65; P=0.006)” [6] so it seems perverse to conclude that there is “no demonstrated benefit” of anticoagulation.
Reference 6 of [1], namely [7], is consistent with our analysis of their references 3 vs 2 above, as it showed that “heparin was more likely to be beneficial in those who were less severely ill at presentation or had lower BMI” [7]. Again, this hardly counts as ‘no demonstrated benefit’.
Reference 7 of [1] involved a study [8] of extremely ill patients, many of whom required mechanical ventilation, again comparing higher vs lower doses of anticoagulant. They conclude “Comparing anticoagulation strategies, a greater proportion of wins occurred with full-dose anticoagulation (12.3%) versus standard-dose prophylactic anticoagulation (6.4%; win ratio, 1.95 {95% CI, 1.08–3.55}; P=0.028)” [8]. As before, it is hard to interpret this as showing ‘no demonstrated benefit’.
Reference 8 of [1] is the work of [9], who concluded [9] “In this randomized clinical trial, therapeutic-dose LMWH reduced major thromboembolism and death compared with institutional standard heparin thromboprophylaxis among inpatients with COVID-19 with very elevated D-dimer levels. The treatment effect was not seen in ICU patients.” Again, the finding is clear; unless nothing is going to save you, anticoagulation helps.
Reference 9 of [1] was by [10] who studied the effectiveness of anticoagulation post-discharge. The conclusion was “In patients at high risk discharged after hospitalisation due to COVID-19, thromboprophylaxis with rivaroxaban 10 mg/day for 35 days improved clinical outcomes compared with no extended thromboprophylaxis” [10]. A clear conclusion in favour of anticoagulation, one might think.
Reference 10 of [1] is a study by the first author [11] but “was terminated after enrollment of 9% of participants because of an event rate lower than anticipated.” [11]
Reference 11 of [1] was a study of enoxaparin [12], also terminated early because of low event rates [13]; the data were rather noisy, albeit “The 90-day incidence of cardiovascular events was 0.9 % in the enoxaparin arm vs. 1.7 % in controls” [12].
Reference 12 of [1] was a study of enoxaparin (on its own) [14], but yet again this was terminated early, with the abstract stating [14] “Following the advice of the Data and Safety Monitoring Board, this study was terminated early due to slow enrolment and a lower-than-expected event rate.”
Finally, reference 13 of [1] was a study of apixaban (on its own) [15]. This study too was abandoned early because of low event numbers. The papers conclusion reads “The incidence of death or thromboembolism was low in this cohort of patients discharged after hospitalization with COVID-19. Because of early enrollment termination, the results were imprecise and the study was inconclusive.” [15].
As with references 10, 11 and 12 of [1] it is hard to understand why an underpowered study could properly be interpreted as an absence of an effect. Citing four studies that could not scientifically or statistically conclude anything (because they were underpowered, and even admitted that they were), then claiming that this showed there was no treatment effect, is simply unprofessional.
We note too that these were all studies of single anticoagulants, which is not in fact what we have been studying and promoting (antiplatelet therapy is also required in order to deal with platelet hyperactivation), and that they were studies of acute COVID, whose demographics (especially regarding gender) are quite different from those of Long COVID. Given the well-established thrombotic elements of acute COVID (even noted by the first author elsewhere [16; 17]) it is equally hard to know what the studies cited as references 2-13 of [1] are supposed to tell us about the microclot phenomena that also demonstrably occur [18-22] in acute COVID.
Curiously, the authors of [1] failed to cite any of the many other and large-scale studies that nonetheless showed a very clear benefit of preexisting or applied anticoagulation in acute COVID, such as [23-28], including cohorts such as those suffering from atrial fibrillation (reference [29] and editorial [30]). Equally, we do note that occasional studies found no effect (e.g. [31])
Coagulation and endothelial abnormalities
The authors of [1] have a section on this, noting that “These findings suggest that ongoing endothelial cell activation is present in some with PASC, and is tied to changes in leukocyte phenotypes, but activation of coagulation and fibrinolysis is not, given normal D-dimer levels”. Here we agree [32-35], but we have never argued that either coagulation of itself or fibrinolysis are activated in PASC. If the authors had bothered to read (or understand) what we have in fact written they would understand why. We have argued consistently that it is not that coagulation is (or has to be) activated any more than normal: it is that the presence of spike protein [36] (or other substances such as LPS [37] in other cases) causes the clotting to be not into ‘normal’ fibrin but into a thermodynamically more stable version that stains with amyloid stains and that – as with other amyloids and prion proteins [38] – is simply not only thermodynamically stabler but is much more resistant to the normal means of its removal via proteases such as plasminogen (see also [39]). Far from fibrinolysis simply ‘not being activated’ we argue that it is inhibited, as this is how proteins containing amyloid structures behave. Additionally, these microclots contain antiplasmin [40] and other molecules [41] that make them even more resistant to fibrinolysis. This lowered fibrinolysis explains entirely why raised D-dimer levels are rarely seen, and are thus irrelevant, in Long COVID; only fibrinolysis generated d-dimer and this process is inhibited. Note too that early variants of the spike protein are themselves unusually amyloidogenic [42], while the omicron version of spike is both far less amyloidogenic and far less virulent [43]; this is an important ‘control’ showing that microclots are on the disease pathway.
Microclots
This question of what are (fibrin amyloid) microclots seems to be at the heart of what causes Connors and Ariëns to have and express their ‘uncertainties’, and is certainly the section in which their statements are at greatest variance with the easily checkable facts. For the avoidance of doubt, we call them fibrin amyloid (or fibrinaloid) microclots because they contain fibrin in an amyloid structure, they are in the micrometre range, and they are clots. (They have since been observed by others who used the amyloid stains [44-47].)
Connors and Ariëns state [1] “PASC is a complex disorder associated with a range of conditions such as chronic fatigue, cognitive disorder, and dizziness, depression and anxiety, some of which are unlikely caused by microclots.” The term ‘unlikely’ is used without any evidence. By contrast, the (temporary) blockage of microcapillaries by microclots, interfering with blood flow and hence inhibiting oxygen transfer to tissues and thence their ability to make ATP, gives a precise, straightforward and mechanistic explanation for each of the symptoms listed. It is the one mechanism that exactly can provide a unitary explanation for many of the symptoms of PASC/ Long COVID. This was all set out in two reviews in 2022 [19; 48] which Connors and Ariëns [1] failed to cite. A more recent extension to the role of the fibrinaloid microclots in generating autoantibodies is now available [49], as is an explanation for their role in POTS [50], in fibromyalgia [51] and even in atrial fibrillation [52].
The following paragraphs will continue with quotations from the microclots section of [1] followed by our explanation, including evidence and citations of published work, and why the comments in [1] are either simply irrelevant or more often completely wrong.
“Microclots have been found in patients with different diseases, and are not specific for PASC.” So what? Inflammatory markers go up in all kinds of inflammatory diseases, as does ferritin [53], including in all those where we observe microclots (see e.g. another uncited review [54]). Glucose is found in healthy individuals as well as in diabetics; quantification is what matters (rather obviously).
“While microclots are microscopic in nature, their composition is variable and does not indicate a clear mechanism of formation. It is not clear whether microclots are indeed the result of blood clotting.” It is not obvious that Connors and Ariëns [1] know anything about their composition (although we have done proteomics [36; 40; 41], as have others since [46]), but the composition of lipoproteins is variable too. The mechanism of formation of microclots is, however, entirely clear, as they contain fibrin, and are indeed the result of clotting (including in vitro with fibrinogen plus thrombin ± LPS or spike). The transition to a thermodynamically stabler amyloid form requires only a tiny amount of catalyst in the form of an amyloid conformer itself. This is all very well established with prions, prionoids, etc [49; 55-57].
“Microclots are detected using an unconventional technique of staining plasma samples with amyloid dyes”. The stains (such as thioflavin T) for amyloid structures are extremely well established [38; 58; 59], and not at all ‘unconventional’. As well as thioflavin T (the classical stain) we have also used oligothiophene ‘Amytracker’ dyes [60-63] that have other desirable properties and are well established as stains for amyloid structures (e.g. [64-70]); we have also correlated the staining with the microclot structures seen in both the electron microscope [71] and in imaging flow cytometry [72].
“Plasma microclots were first reported in patients with type 2 diabetes in 2017”. Not true. While the first report that they were in fact amyloid in nature, as judged by optical microscopy was in 2016 [37], Pretorius had been studying them by electron microscopy since 2006 (e.g. [73]) where they were sometimes referred to as ‘dense matted deposits’ [74-76]. Other early studies include references [77-86]. In other words, these discoveries have been being made for over 15 years.
“It currently remains unknown if similar microclots occur in plasma samples of patients with other thrombotic disorders”. Given the claim above that they occur in other diseases besides long COVID, one might have thought that the authors of [1] might have noted that the other syndromes where we have noted them are indeed vascular or thrombotic, not least because one of the authors actually published to this effect [87].
“These microclots do not appear to precipitate alongside blood cells during centrifugation when plasma is prepared. It is possible that microclots have lower density, however, an alternative explanation is that they form after the plasma is taken”. If the microclots formed after the plasma has been taken, they would have to be very clever and know that they should do this only from patients and not controls. We do not consider this reasonable (I’m putting this politely).
“The molecular or cellular mechanisms involved in the formation of microclots are also unknown.” Not true. See above for any of the many papers cited showing how a number of substances can induce polymerisation of fibrinogen into one or more amyloid forms.
“Microclot aggregates have also been termed amyloid microclots, suggesting they could be the result of a different process such as amyloidosis.” With one rare hereditary exception [88; 89], the established amyloidoses [90] do not include normal fibrin(ogen) which is what we discovered could in fact become amyloid. It is possible that the authors of [1] did not recognise that ‘amyloid’ here simply refers to a kind of protein structure, which we know to involve crossed-beta sheets [91-93] and that this is what is stained by the amyloid stains [58].
“However, more data are needed to demonstrate their relative resistance to fibrinolysis using in-vitro systems”. Data such as the resistance to trypsinisation already demonstrated in [36; 40; 41], presumably?
“in-vivo data demonstrating that anticoagulant treatments and plasma exchange reduce the presence or formation of microclots, and that doing so benefits patients with PASC, are needed from well controlled studies before inferences are made regarding possible treatments aimed at reducing microclots”. Here the non-sequiturs get so twisted upon themselves that they make even less sense than the rest of the article. It appears to ask for anticoagulant treatments or plasmapheresis (we suspect they mean H.E.L.P. apheresis but that was equally unclear in another paper [94]) to be studied before such treatments are administered. Fortunately at least one has been [95].
Summary
The summary covers many areas including ME/CFS (where hyperactivated platelets and microclots have also been demonstrated [96; 97], the first ever proper biomarker) and vaccination and antivirals, plus (unknown and unspecified) ‘treatments aimed at suppressing activation of latent viruses’.
In contrast to these speculations, what we have provided is a well-established and self-consistent set of observations, that include mechanistic explanations (Fig 1), entirely fulfilling the Bradford Hill criteria [98] of whether agent X (microclots) is involved the aetiology of disease Y (Long COVID). The microclots are overwhelming candidates since they appear far more in the cases than the controls (much as with LDL cholesterol in certain kinds of heart disease), when formed by the much less virulent omicron spike they are far less prevalent (an important control), proper anticoagulation with two or three dugs results in clinical benefit, and removing them with nutraceutical enzymes also provides therapeutic opportunities [19; 48] (see also most recently [99]).
We subscribe to the scientific principle of explanatory coherence [100-105]; this says that if a series of ostensibly unrelated facts all point at the same mechanism, it thereby strengthens the likelihood of that mechanism being true. In our view, when studied over the full and extensive range of evidence presented by us and by others, and not by flagrantly selective citations of underpowered experiments, the evidence (i.e. data plus correct interpretation) shows the aetiology of the role of microclots in Long COVID, along with platelet hyperactivation (not discussed here, but in our papers), to be crystal clear.
We do not have real control over what others choose to believe [106] or to publish, even when it has been shown to be false (yet another piece attacking us has just appeared, again repeating things shown to be false) and will be rebutted in detail in a similar manner, but journals and their Editors do have a responsibility to ensure that when inaccurate claims are made they allow the publication of full rebuttals that include the facts. In the absence of this, a blog provides the best mechanism. It is an odd strategy in science to claim there is no evdence for something by simply ignoring it, though I recognise that this strategy is not unique to this area of science (e.g. [Kell, 2021 #83151;Kell, 2021 #81314]), so I’ll continue to call out those who think it is OK until they stop doing it.
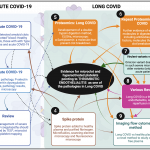
A summary of the development of the microclot theory, written as part of the original rebuttal sent to the journal in 2023, is given in the Figure (click for full size).
Figure. The timeline for microclot discovery in Long COVID and ultimately the hypothesis that Long COVID is a thrombotic endothelialitis. After our first review of Long COVID was written in 2020 when we discussed the clotting and bleeding phenomenon in acute Covid-19 [18]. (1) First blood analysis of acute COVID-19 where we found amyloid deposits in “unclotted” blood [22]. (2) A paper followed where we looked at platelets, and in particular the thrombotic marker, P-selectin [107]. (3) A review followed where we suggested a platelet and microclot grading system and discussed an acute COVID-19 treatment regimen that our clinical collaborator followed [20]. (4) We added spike protein to purified fibrinogen and plasma form healthy individuals and showed significant clotting aggregates using scanning electron microscopy, very preliminary proteomics and microfluidics [36]. (5) We received the first reports of long COVID and collected the first blood samples form these patients in South Africa, a first proteomics analysis followed that showed fibrinolytic-resistant plasma deposits. The term “microclots” were first used. Alpha-2 antiplasmin were found in digested microclots [40]. (6) A repeat proteomics study followed with a larger cohort of participants [41]. (7) A US collaborator team, under the lead of Dr Mark Walsh, co-authored with us a paper that showed that Omicron was both less amyloidogenic and less virulent than older more virulent variants [43] (implying that microclots are on the disease pathway). (8) Various reviews followed to discuss the thrombotic nature of Long COVID and its consequences, and that we now suggest that the disease is a thrombotic endothelialitis [19; 33; 48; 49]. (9) Most recently, we developed the first imaging flow cytometry methods to quantify microclots in plasma samples [72].
References
[1] Connors, J. M. & Ariëns, R. A. S. (2023). Uncertainties about the Roles of Anticoagulation and Microclots in Post-acute Sequelae of SARS-CoV-2 Infection. J Thromb Hemost.
[2] Kell, D. B., Khan, M. A., Laubscher, G. J. & Pretorius, E. (2024). Uncertainties about the roles of anticoagulation and microclots in postacute sequelae of SARS-CoV-2 infection: comment from Kell et al. J Thromb Haemost 22, 565-568.
[3] Goligher, E. C., Bradbury, C. A., McVerry, B. J., Lawler, P. R., Berger, J. S., Gong, M. N., Carrier, M., Reynolds, H. R., Kumar, A., Turgeon, A. F., Kornblith, L. Z., Kahn, S. R., Marshall, J. C., Kim, K. S., Houston, B. L., Derde, L. P. G., Cushman, M., Tritschler, T., Angus, D. C., Godoy, L. C., McQuilten, Z., Kirwan, B. A., Farkouh, M. E., Brooks, M. M., Lewis, R. J., Berry, L. R., Lorenzi, E., Gordon, A. C., Ahuja, T., Al-Beidh, F., Annane, D., Arabi, Y. M., Aryal, D., Baumann Kreuziger, L., Beane, A., Bhimani, Z., Bihari, S., Billett, H. H., Bond, L., Bonten, M., Brunkhorst, F., Buxton, M., Buzgau, A., Castellucci, L. A., Chekuri, S., Chen, J. T., Cheng, A. C., Chkhikvadze, T., Coiffard, B., Contreras, A., Costantini, T. W., de Brouwer, S., Detry, M. A., Duggal, A., Dzavik, V., Effron, M. B., Eng, H. F., Escobedo, J., Estcourt, L. J., Everett, B. M., Fergusson, D. A., Fitzgerald, M., Fowler, R. A., Froess, J. D., Fu, Z., Galanaud, J. P., Galen, B. T., Gandotra, S., Girard, T. D., Goodman, A. L., Goossens, H., Green, C., Greenstein, Y. Y., Gross, P. L., Haniffa, R., Hegde, S. M., Hendrickson, C. M., Higgins, A. M., Hindenburg, A. A., Hope, A. A., Horowitz, J. M., Horvat, C. M., Huang, D. T., Hudock, K., Hunt, B. J., Husain, M., Hyzy, R. C., Jacobson, J. R., Jayakumar, D., Keller, N. M., Khan, A., Kim, Y., Kindzelski, A., King, A. J., Knudson, M. M., Kornblith, A. E., Kutcher, M. E., Laffan, M. A., Lamontagne, F., Le Gal, G., et al. (2021). Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med 385, 777-789.
[4] Lawler, P. R., Goligher, E. C., Berger, J. S., Neal, M. D., McVerry, B. J., Nicolau, J. C., Gong, M. N., Carrier, M., Rosenson, R. S., Reynolds, H. R., Turgeon, A. F., Escobedo, J., Huang, D. T., Bradbury, C. A., Houston, B. L., Kornblith, L. Z., Kumar, A., Kahn, S. R., Cushman, M., McQuilten, Z., Slutsky, A. S., Kim, K. S., Gordon, A. C., Kirwan, B. A., Brooks, M. M., Higgins, A. M., Lewis, R. J., Lorenzi, E., Berry, S. M., Berry, L. R., Aday, A. W., Al-Beidh, F., Annane, D., Arabi, Y. M., Aryal, D., Baumann Kreuziger, L., Beane, A., Bhimani, Z., Bihari, S., Billett, H. H., Bond, L., Bonten, M., Brunkhorst, F., Buxton, M., Buzgau, A., Castellucci, L. A., Chekuri, S., Chen, J. T., Cheng, A. C., Chkhikvadze, T., Coiffard, B., Costantini, T. W., de Brouwer, S., Derde, L. P. G., Detry, M. A., Duggal, A., Dzavik, V., Effron, M. B., Estcourt, L. J., Everett, B. M., Fergusson, D. A., Fitzgerald, M., Fowler, R. A., Galanaud, J. P., Galen, B. T., Gandotra, S., Garcia-Madrona, S., Girard, T. D., Godoy, L. C., Goodman, A. L., Goossens, H., Green, C., Greenstein, Y. Y., Gross, P. L., Hamburg, N. M., Haniffa, R., Hanna, G., Hanna, N., Hegde, S. M., Hendrickson, C. M., Hite, R. D., Hindenburg, A. A., Hope, A. A., Horowitz, J. M., Horvat, C. M., Hudock, K., Hunt, B. J., Husain, M., Hyzy, R. C., Iyer, V. N., Jacobson, J. R., Jayakumar, D., Keller, N. M., Khan, A., Kim, Y., Kindzelski, A. L., King, A. J., Knudson, M. M., Kornblith, A. E., Krishnan, V., et al. (2021). Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med 385, 790-802.
[5] Sadeghipour, P., Talasaz, A. H., Rashidi, F., Sharif-Kashani, B., Beigmohammadi, M. T., Farrokhpour, M., Sezavar, S. H., Payandemehr, P., Dabbagh, A., Moghadam, K. G., Jamalkhani, S., Khalili, H., Yadollahzadeh, M., Riahi, T., Rezaeifar, P., Tahamtan, O., Matin, S., Abedini, A., Lookzadeh, S., Rahmani, H., Zoghi, E., Mohammadi, K., Sadeghipour, P., Abri, H., Tabrizi, S., Mousavian, S. M., Shahmirzaei, S., Bakhshandeh, H., Amin, A., Rafiee, F., Baghizadeh, E., Mohebbi, B., Parhizgar, S. E., Aliannejad, R., Eslami, V., Kashefizadeh, A., Kakavand, H., Hosseini, S. H., Shafaghi, S., Ghazi, S. F., Najafi, A., Jimenez, D., Gupta, A., Madhavan, M. V., Sethi, S. S., Parikh, S. A., Monreal, M., Hadavand, N., Hajighasemi, A., Maleki, M., Sadeghian, S., Piazza, G., Kirtane, A. J., Van Tassell, B. W., Dobesh, P. P., Stone, G. W., Lip, G. Y. H., Krumholz, H. M., Goldhaber, S. Z. & Bikdeli, B. (2021). Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA 325, 1620-1630.
[6] Sholzberg, M., Tang, G. H., Rahhal, H., AlHamzah, M., Kreuziger, L. B., Ainle, F. N., Alomran, F., Alayed, K., Alsheef, M., AlSumait, F., Pompilio, C. E., Sperlich, C., Tangri, S., Tang, T., Jaksa, P., Suryanarayan, D., Almarshoodi, M., Castellucci, L. A., James, P. D., Lillicrap, D., Carrier, M., Beckett, A., Colovos, C., Jayakar, J., Arsenault, M. P., Wu, C., Doyon, K., Andreou, E. R., Dounaevskaia, V., Tseng, E. K., Lim, G., Fralick, M., Middeldorp, S., Lee, A. Y. Y., Zuo, F., da Costa, B. R., Thorpe, K. E., Negri, E. M., Cushman, M., Jüni, P. & Rapid trial investigators. (2021). Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 375, n2400.
[7] Goligher, E. C., Lawler, P. R., Jensen, T. P., Talisa, V., Berry, L. R., Lorenzi, E., McVerry, B. J., Chang, C. H., Leifer, E., Bradbury, C., Berger, J., Hunt, B. J., Castellucci, L. A., Kornblith, L. Z., Gordon, A. C., McArthur, C., Webb, S., Hochman, J., Neal, M. D., Zarychanski, R., Berry, S., Angus, D. C., Remap-Cap, A. & Investigators, A. C.-a. (2023). Heterogeneous Treatment Effects of Therapeutic-Dose Heparin in Patients Hospitalized for COVID-19. JAMA 329, 1066-1077.
[8] Bohula, E. A., Berg, D. D., Lopes, M. S., Connors, J. M., Babar, I., Barnett, C. F., Chaudhry, S. P., Chopra, A., Ginete, W., Ieong, M. H., Katz, J. N., Kim, E. Y., Kuder, J. F., Mazza, E., McLean, D., Mosier, J. M., Moskowitz, A., Murphy, S. A., O’Donoghue, M. L., Park, J. G., Prasad, R., Ruff, C. T., Shahrour, M. N., Sinha, S. S., Wiviott, S. D., Van Diepen, S., Zainea, M., Baird-Zars, V., Sabatine, M. S., Morrow, D. A. & Covid-Pact Investigators. (2022). Anticoagulation and Antiplatelet Therapy for Prevention of Venous and Arterial Thrombotic Events in Critically Ill Patients With COVID-19: COVID-PACT. Circulation 146, 1344-1356.
[9] Spyropoulos, A. C., Goldin, M., Giannis, D., Diab, W., Wang, J., Khanijo, S., Mignatti, A., Gianos, E., Cohen, M., Sharifova, G., Lund, J. M., Tafur, A., Lewis, P. A., Cohoon, K. P., Rahman, H., Sison, C. P., Lesser, M. L., Ochani, K., Agrawal, N., Hsia, J., Anderson, V. E., Bonaca, M., Halperin, J. L., Weitz, J. I. & Investigators, H.-C. (2021). Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial. JAMA Intern Med 181, 1612-1620.
[10] Ramacciotti, E., Barile Agati, L., Calderaro, D., Aguiar, V. C. R., Spyropoulos, A. C., de Oliveira, C. C. C., Lins Dos Santos, J., Volpiani, G. G., Sobreira, M. L., Joviliano, E. E., Bohatch Junior, M. S., da Fonseca, B. A. L., Ribeiro, M. S., Dusilek, C., Itinose, K., Sanches, S. M. V., de Almeida Araujo Ramos, K., de Moraes, N. F., Tierno, P., de Oliveira, A., Tachibana, A., Chate, R. C., Santos, M. V. B., de Menezes Cavalcante, B. B., Moreira, R. C. R., Chang, C., Tafur, A., Fareed, J., Lopes, R. D. & investigators, M. (2022). Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): an open-label, multicentre, randomised, controlled trial. Lancet 399, 50-59.
[11] Connors, J. M., Brooks, M. M., Sciurba, F. C., Krishnan, J. A., Bledsoe, J. R., Kindzelski, A., Baucom, A. L., Kirwan, B. A., Eng, H., Martin, D., Zaharris, E., Everett, B., Castro, L., Shapiro, N. L., Lin, J. Y., Hou, P. C., Pepine, C. J., Handberg, E., Haight, D. O., Wilson, J. W., Majercik, S., Fu, Z., Zhong, Y., Venugopal, V., Beach, S., Wisniewski, S., Ridker, P. M. & Investigators, A.-B. (2021). Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA 326, 1703-1712.
[12] Voci, D., Gotschi, A., Held, U., Bingisser, R., Colucci, G., Duerschmied, D., Fumagalli, R. M., Gerber, B., Hasse, B., Keller, D. I., Konstantinides, S. V., Mach, F., Rampini, S. K., Righini, M., Robert-Ebadi, H., Rosemann, T., Roth-Zetzsche, S., Sebastian, T., Simon, N. R., Spirk, D., Stortecky, S., Vaisnora, L., Kucher, N., Barco, S. & OVID investigators. (2023). Enoxaparin for outpatients with COVID-19: 90-day results from the randomised, open-label, parallel-group, multinational, phase III OVID trial. Thromb Res 221, 157-163.
[13] Barco, S., Voci, D., Held, U., Sebastian, T., Bingisser, R., Colucci, G., Duerschmied, D., Frenk, A., Gerber, B., Gotschi, A., Konstantinides, S. V., Mach, F., Robert-Ebadi, H., Rosemann, T., Simon, N. R., Spechbach, H., Spirk, D., Stortecky, S., Vaisnora, L., Righini, M., Kucher, N. & OVID investigators. (2022). Enoxaparin for primary thromboprophylaxis in symptomatic outpatients with COVID-19 (OVID): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet Haematol 9, e585-e593.
[14] Cools, F., Virdone, S., Sawhney, J., Lopes, R. D., Jacobson, B., Arcelus, J. I., Hobbs, F. D. R., Gibbs, H., Himmelreich, J. C. L., MacCallum, P., Schellong, S., Haas, S., Turpie, A. G. G., Ageno, W., Rocha, A. T., Kayani, G., Pieper, K., Kakkar, A. K. & investigators, E. (2022). Thromboprophylactic low-molecular-weight heparin versus standard of care in unvaccinated, at-risk outpatients with COVID-19 (ETHIC): an open-label, multicentre, randomised, controlled, phase 3b trial. Lancet Haematol 9, e594-e604.
[15] Wang, T. Y., Wahed, A. S., Morris, A., Kreuziger, L. B., Quigley, J. G., Lamas, G. A., Weissman, A. J., Lopez-Sendon, J., Knudson, M. M., Siegal, D. M., Kasthuri, R. S., Alexander, A. J., Wahid, L., Atassi, B., Miller, P. J., Lawson, J. W., Patel, B., Krishnan, J. A., Shapiro, N. L., Martin, D. E., Kindzelski, A. L., Leifer, E. S., Joo, J., Lyu, L., Pennella, A., Everett, B. M., Geraci, M. W., Anstrom, K. J., Ortel, T. L. & Group, A.-C. S. (2023). Effect of Thromboprophylaxis on Clinical Outcomes After COVID-19 Hospitalization. Ann Intern Med 176, 515-523.
[16] Connors, J. M. & Levy, J. H. (2020). COVID-19 and its implications for thrombosis and anticoagulation. Blood 135, 2033-2040.
[17] Connors, J. M. & Levy, J. H. (2020). Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 18, 1559-1561.
[18] Grobler, C., Maphumulo, S. C., Grobbelaar, L. M., Bredenkamp`, J., Laubscher, J., Lourens, P. J., Steenkamp, J., Kell, D. B. & Pretorius, E. (2020). COVID-19: The Rollercoaster of Fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci 21, 5168.
[19] Kell, D. B., Laubscher, G. J. & Pretorius, E. (2022). A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J 479, 537-559.
[20] Laubscher, G. J., Lourens, P. J., Venter, C., Kell, D. B. & Pretorius, E. (2021). TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med 10, 5381.
[21] Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. & Kell, D. B. (2020). Prevalence of amyloid blood clots in COVID-19 plasma. medRxiv, 2020.07.28.20163543v1.
[22] Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. & Kell, D. B. (2020). Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report. Cardiovasc Diabetol 19, 193.
[23] Tang, N., Bai, H., Chen, X., Gong, J., Li, D. & Sun, Z. (2020). Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18, 1094-1099.
[24] Iaccarino, G., Grassi, G., Borghi, C., Grassi, D., Mancusi, C., Muiesan, M. L., Salvetti, M., Volpe, M. & Ferri, C. (2021). Preexisting Oral Anticoagulant Therapy Ameliorates Prognosis in Hospitalized COVID-19 Patients. Front Cardiovasc Med 8, 633878.
[25] Chocron, R., Galand, V., Cellier, J., Gendron, N., Pommier, T., Bory, O., Khider, L., Trimaille, A., Goudot, G., Weizman, O., Alsac, J. M., Geneste, L., Schmeltz, A., Panagides, V., Philippe, A., Marsou, W., Ben Abdallah, I., Deney, A., El Batti, S., Attou, S., Juvin, P., Delmotte, T., Messas, E., Pezel, T., Planquette, B., Duceau, B., Gaussem, P., Sutter, W., Sanchez, O., Waldman, V., Diehl, J. L., Mirault, T., Bonnet, G., Cohen, A., Smadja, D. M. & Critical Covid-France Investigators. (2021). Anticoagulation Before Hospitalization Is a Potential Protective Factor for COVID-19: Insight From a French Multicenter Cohort Study. J Am Heart Assoc 10, e018624.
[26] Rieder, M., Gauchel, N., Kaier, K., Jakob, C., Borgmann, S., Classen, A. Y., Schneider, J., Eberwein, L., Lablans, M., Ruthrich, M., Dolff, S., Wille, K., Haselberger, M., Heuzeroth, H., Bode, C., von Zur Muhlen, C., Rieg, S. & Duerschmied, D. (2022). Pre-medication with oral anticoagulants is associated with better outcomes in a large multinational COVID-19 cohort with cardiovascular comorbidities. Clin Res Cardiol 111, 322-332.
[27] Wong, A. Y., Tomlinson, L., Brown, J. P., Elson, W., Walker, A. J., Schultze, A., Morton, C. E., Evans, D., Inglesby, P., MacKenna, B., Bhaskaran, K., Rentsch, C. T., Powell, E., Williamson, E., Croker, R., Bacon, S., Hulme, W., Bates, C., Curtis, H. J., Mehrkar, A., Cockburn, J., McDonald, H. I., Mathur, R., Wing, K., Forbes, H., Eggo, R. M., Evans, S. J., Smeeth, L., Goldacre, B. & Douglas, I. J. (2022). Association between oral anticoagulants and COVID-19-related outcomes: a population-based cohort study. Br J Gen Pract 72, e456-e463.
[28] Zapata-Cachafeiro, M., Prieto-Campo, Á., Portela-Romero, M., Carracedo-Martínez, E., Lema-Oreiro, M., Piñeiro-Lamas, M., Chaudhuri, S., Salgado-Barreira, Á. & Figueiras, A. (2023). Effect of Previous Anticoagulant Treatment on Risk of COVID-19. Drug Saf 46, 273-281.
[29] Handy, A., Banerjee, A., Wood, A. M., Dale, C., Sudlow, C. L. M., Tomlinson, C., Bean, D., Thygesen, J. H., Mizani, M. A., Katsoulis, M., Takhar, R., Hollings, S., Denaxas, S., Walker, V., Dobson, R., Sofat, R. & CVD-COVID-UK Consortium. (2022). Evaluation of antithrombotic use and COVID-19 outcomes in a nationwide atrial fibrillation cohort. Heart, in press.
[30] Raatikainen, P. & Lassila, R. (2022). COVID-19: another reason for anticoagulation in patients with atrial fibrillation. Heart 108, 902-904.
[31] Altaraihi, S., Kamstrup, P., Eklof, J., Dyrby Johansen, N., Biering-Sorensen, T., Sivapalan, P. & Jensen, J. U. (2023). Treatment with prophylactic oral anticoagulants and the risk of mortality in COVID-19 patients: a nationwide cohort study. ERJ Open Res 9.
[32] Turner, S., Naidoo, C., Usher, T., Kruger, A., Venter, C., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2022). Increased levels of inflammatory molecules in blood of Long COVID patients point to thrombotic endotheliitis. medRxiv, 2022.10.13.22281055.
[33] Turner, S., Khan, M. A., Putrino, D., Woodcock, A., Kell, D. B. & Pretorius, E. (2023). Long COVID: pathophysiological factors and abnormal coagulation. Trends Endocrinol Metab 34, 321-344.
[34] Turner, S., Naidoo, C. A., Usher, T. J., Kruger, A., Venter, C., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2023). Increased Levels of Inflammatory and Endothelial Biomarkers in Blood of Long COVID Patients Point to Thrombotic Endothelialitis. Semin Thromb Hemost.
[35] Laubscher, G. J., Khan, M. A., Venter, C., Pretorius, J. H., Kell, D. B. & Pretorius, E. (2023). Treatment of Long COVID symptoms with triple anticoagulant therapy. https://www.researchsquare.com/article/rs-2697680/v1.
[36] Grobbelaar, L. M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B. & Pretorius, E. (2021). SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep 41, BSR20210611.
[37] Pretorius, E., Mbotwe, S., Bester, J., Robinson, C. J. & Kell, D. B. (2016). Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface 123, 20160539.
[38] Kell, D. B. & Pretorius, E. (2017). Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr Biophys Mol Biol 123, 16-41.
[39] Kell, D. B. & Pretorius, E. (2015). The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integr Biol 7, 24-52.
[40] Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Steenkamp, J. & Kell, D. B. (2021). Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 20, 172.
[41] Kruger, A., Vlok, M., Turner, S., Venter, C., Laubscher, G. J., Kell, D. B. & Pretorius, E. (2022). Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol 21, 190.
[42] Nyström, S. & Hammarström, P. (2022). Amyloidogenesis of SARS-CoV-2 Spike Protein. J Am Chem Soc 144, 8945-8950.
[43] Grobbelaar, L. M., Kruger, A., Venter, C., Burger, E. M., Laubscher, G. J., Maponga, T. G., Kotze, M. J., Kwaan, H. C., Miller, J. B., Fulkerson, D., Huff, W., Chang, E., Wiarda, G., Bunch, C. M., Walsh, M. M., Raza, S., Zamlut, M., Moore, H. B., Moore, E. E., Neal, M. D., Kell, D. B. & Pretorius, E. (2022). Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost 48, 858-868.
[44] Appelman, B., Charlton, B. T., Goulding, R. P., Kerkhoff, T. J., Breedveld, E. A., Noort, W., Offringa, C., Bloemers, F. W., van Weeghel, M., Schomakers, B. V., Coelho, P., Posthuma, J. J., Aronica, E., Joost Wiersinga, W., van Vugt, M. & Wüst, R. C. I. (2024). Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun 15, 17.
[45] Dalton, C. F., de Oliveira, M. I. R., Stafford, P., Peake, N., Kane, B., Higham, A., Singh, D., Jackson, N., Davies, H., Price, D., Duncan, R., Tattersall, N., Barnes, A. & Smith, D. P. (2024). Increased fibrinaloid microclot counts in platelet-poor plasma are associated with Long COVID. medRxiv, 2024.04.04.24305318.
[46] Schofield, J., Abrams, S. T., Jenkins, R., Lane, S., Wang, G. & Toh, C. H. (2024). Amyloid-fibrinogen aggregates (“microclots”) predict risks of Disseminated Intravascular Coagulation and mortality. Blood Adv 8, 2499-2508.
[47] Okuducu, Y. K., Boribong, B., Ellett, F., Hajizadeh, S., VanElzakker, M., Haas, W., Pillai, S., Fasano, A., Irimia, D. & Yonker, L. (2024). Evidence Circulating Microclots and Activated Platelets Contribute to Hyperinflammation Within Pediatric Post Acute Sequala of COVID. Am J Respir Crit Care Med 209, A2247.
[48] Kell, D. B. & Pretorius, E. (2022). The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J 479, 1653-1708.
[49] Kell, D. B. & Pretorius, E. (2023). Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? Biochem J, in press.
[50] Kell, D. B., Khan, M. A., Kane, B., Lip, G. Y. H. & Pretorius, E. (2024). Possible Role of Fibrinaloid Microclots in Postural Orthostatic Tachycardia Syndrome (POTS): Focus on Long COVID. J Pers Med 14, 170.
[51] Kell, D. B. & Pretorius, E. (2024). Potential roles of fibrinaloid microclots in fibromyalgia syndrome. OSF preprint, https://osf.io/9e2y5/.
[52] Kell, D. B., Lip, G. Y. H. & Pretorius, E. (2024). Fibrinaloid Microclots and Atrial Fibrillation. Biomedicines 12, 891.
[53] Kell, D. B. & Pretorius, E. (2014). Serum ferritin is an important disease marker, and is mainly a leakage product from damaged cells. Metallomics 6, 748-773.
[54] Kell, D. B. & Pretorius, E. (2018). No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev 93, 1518-1557.
[55] Aguzzi, A. & Lakkaraju, A. K. K. (2016). Cell Biology of Prions and Prionoids: A Status Report. Trends Cell Biol 26, 40-51.
[56] Ashe, K. H. & Aguzzi, A. (2013). Prions, prionoids and pathogenic proteins in Alzheimer disease. Prion 7, 55-9.
[57] Liberski, P. P. (2014). Prion, prionoids and infectious amyloid. Parkinsonism Relat Disord 20 Suppl 1, S80-4.
[58] Biancalana, M. & Koide, S. (2010). Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta 1804, 1405-12.
[59] Khurana, R., Coleman, C., Ionescu-Zanetti, C., Carter, S. A., Krishna, V., Grover, R. K., Roy, R. & Singh, S. (2005). Mechanism of thioflavin T binding to amyloid fibrils. J Struct Biol 151, 229-38.
[60] Pretorius, E., Page, M. J., Engelbrecht, L., Ellis, G. C. & Kell, D. B. (2017). Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol 16, 141.
[61] Pretorius, E., Page, M. J., Hendricks, L., Nkosi, N. B., Benson, S. R. & Kell, D. B. (2018). Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface 15, 20170941.
[62] Adams, B., Nunes, J. M., Page, M. J., Roberts, T., Carr, J., Nell, T. A., Kell, D. B. & Pretorius, E. (2019). Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. Front Ag Neurosci 11, 210.
[63] Page, M. J., Thomson, G. J. A., Nunes, J. M., Engelbrecht, A. M., Nell, T. A., de Villiers, W. J. S., de Beer, M. C., Engelbrecht, L., Kell, D. B. & Pretorius, E. (2019). Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Sci Rep 9, 3102.
[64] Åslund, A., Sigurdson, C. J., Klingstedt, T., Grathwohl, S., Bolmont, T., Dickstein, D. L., Glimsdal, E., Prokop, S., Lindgren, M., Konradsson, P., Holtzman, D. M., Hof, P. R., Heppner, F. L., Gandy, S., Jucker, M., Aguzzi, A., Hammarström, P. & Nilsson, K. P. R. (2009). Novel pentameric thiophene derivatives for in vitro and in vivo optical imaging of a plethora of protein aggregates in cerebral amyloidoses. ACS Chem Biol 4, 673-84.
[65] Brelstaff, J., Ossola, B., Neher, J. J., Klingstedt, T., Nilsson, K. P. R., Goedert, M., Spillantini, M. G. & Tolkovsky, A. M. (2015). The fluorescent pentameric oligothiophene pFTAA identifies filamentous tau in live neurons cultured from adult P301S tau mice. Front Neurosci 9, 184.
[66] Hammarström, P., Simon, R., Nystrom, S., Konradsson, P., Åslund, A. & Nilsson, K. P. R. (2010). A fluorescent pentameric thiophene derivative detects in vitro-formed prefibrillar protein aggregates. Biochemistry 49, 6838-45.
[67] Nilsson, K. P., Lindgren, M. & Hammarström, P. (2012). A pentameric luminescent-conjugated oligothiophene for optical imaging of in vitro-formed amyloid fibrils and protein aggregates in tissue sections. Methods Mol Biol 849, 425-34.
[68] Rasmussen, J., Mahler, J., Beschorner, N., Kaeser, S. A., Häsler, L. M., Baumann, F., Nyström, S., Portelius, E., Blennow, K., Lashley, T., Fox, N. C., Sepulveda-Falla, D., Glatzel, M., Oblak, A. L., Ghetti, B., Nilsson, K. P. R., Hammarström, P., Staufenbiel, M., Walker, L. C. & Jucker, M. (2017). Amyloid polymorphisms constitute distinct clouds of conformational variants in different etiological subtypes of Alzheimer’s disease. Proc Natl Acad Sci U S A 114, 13018-13023.
[69] Sjölander, D., Röcken, C., Westermark, P., Westermark, G. T., Nilsson, K. P. R. & Hammarström, P. (2016). Establishing the fluorescent amyloid ligand h-FTAA for studying human tissues with systemic and localized amyloid. Amyloid 23, 98-108.
[70] Stepanchuk, A., Tahir, W., Nilsson, K. P. R., Schatzl, H. M. & Stys, P. K. (2021). Early detection of prion protein aggregation with a fluorescent pentameric oligothiophene probe using spectral confocal microscopy. J Neurochem 156, 1033-1048.
[71] de Waal, G. M., Engelbrecht, L., Davis, T., de Villiers, W. J. S., Kell, D. B. & Pretorius, E. (2018). Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson’s Disease, Alzheimer’s Disease and Type 2 Diabetes Mellitus. Sci Rep 8, 16798.
[72] Turner, S., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2023). Rapid flow cytometric analysis of fibrin amyloid microclots in Long COVID. Preprint at https://www.researchsquare.com/article/rs-2731434/v1, submitted.
[73] Pretorius, E., Briedenhann, S., Marx, J. & Franz, R. C. (2006). Structural changes in the fibrin network of a Pretoria family with dysfibrinogenemia: a scanning electron microscopical study. Ultrastruct Pathol 30, 167-76.
[74] Swanepoel, A. C., Lindeque, B. G., Swart, P. J., Abdool, Z. & Pretorius, E. (2014). Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: a qualitative investigation. Microsc Res Tech 77, 594-601.
[75] Swanepoel, A. C., Lindeque, B. G., Swart, P. J., Abdool, Z. & Pretorius, E. (2014). Ultrastructural changes of fibrin networks during three phases of pregnancy: a qualitative investigation. Microsc Res Tech 77, 602-608.
[76] Swanepoel, A. C., Visagie, A., de Lange, Z., Emmerson, O., Nielsen, V. G. & Pretorius, E. (2016). The clinical relevance of altered fibrinogen packaging in the presence of 17beta-estradiol and progesterone. Thromb Res 146, 23-34.
[77] Pretorius, E., Swanepoel, A. C., Oberholzer, H. M., van der Spuy, W. J., Duim, W. & Wessels, P. F. (2011). A descriptive investigation of the ultrastructure of fibrin networks in thrombo-embolic ischemic stroke. J Thromb Thrombolysis 31, 507-13.
[78] Pretorius, E., Steyn, H., Engelbrecht, M., Swanepoel, A. C. & Oberholzer, H. M. (2011). Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul Fibrinolysis 22, 696-700.
[79] Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. & Soma, P. (2011). Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes. Blood Coagul Fibrinol 22, 463-7.
[80] Pretorius, E., Olivier, J., Oberholzer, H. M. & Van der Spuy, W. J. (2011). Scanning electron microscopy investigation of fibrin networks after thermal injury. Onderstepoort J Vet Res 78, 244.
[81] Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. & Soma, P. (2012). Scanning electron microscopy of fibrin networks in rheumatoid arthritis: a qualitative analysis. Rheumatol Int 32, 1611-5.
[82] Pretorius, E., Vermeulen, N., Bester, J. & Lipinski, B. (2013). Novel use of scanning electron microscopy for detection of iron-induced morphological changes in human blood. Microsc Res Tech 76, 268-271.
[83] Pretorius, E. & Lipinski, B. (2013). Differences in morphology of fibrin clots induced with thrombin and ferric ions and its pathophysiological consequences. Heart Lung Circ 22, 447-449.
[84] Pretorius, E., Bester, J., Vermeulen, N., Lipinski, B., Gericke, G. S. & Kell, D. B. (2014). Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents. PLoS One 9, e85271.
[85] Pretorius, E., du Plooy, J., Soma, P. & Gasparyan, A. Y. (2014). An ultrastructural analysis of platelets, erythrocytes, white blood cells, and fibrin network in systemic lupus erythematosus. Rheumatol Int 34, 1005-1009.
[86] Pretorius, E. & Kell, D. B. (2014). Diagnostic morphology: biophysical indicators for iron-driven inflammatory diseases. Integrative Biol 6, 486-510.
[87] Baker, S. R., Halliday, G., Ząbczyk, M., Alkarithi, G., Macrae, F. L., Undas, A., Hunt, B. J. & Ariëns, R. A. S. (2023). Plasma from patients with pulmonary embolism show aggregates that reduce after anticoagulation. Commun Med (Lond) 3, 12.
[88] Gillmore, J. D., Lachmann, H. J., Rowczenio, D., Gilbertson, J. A., Zeng, C. H., Liu, Z. H., Li, L. S., Wechalekar, A. & Hawkins, P. N. (2009). Diagnosis, pathogenesis, treatment, and prognosis of hereditary fibrinogen A alpha-chain amyloidosis. J Am Soc Nephrol 20, 444-51.
[89] Gillmore, J. D., Lachmann, H. J., Wechalekar, A. & Hawkins, P. N. (2010). Hereditary fibrinogen A alpha-chain amyloidosis: clinical phenotype and role of liver transplantation. Blood 115, 4313; author reply 4314-5.
[90] Palladini, G. & Merlini, G. (2013). Systemic amyloidoses: what an internist should know. Eur J Intern Med 24, 729-39.
[91] Morris, K. L. & Serpell, L. C. (2013). From Molecular to Supramolecular Amyloid Structures: Contributions from Fiber Diffraction and Electron Microscopy. In Amyloid Fibrils and Prefibrillar Aggregates: Molecular and Biological Properties (ed. D. E. Otzen), pp. 63-84. Wiley-VCH, Weinheim.
[92] Ivanova, M. I., Lin, Y., Lee, Y. H., Zheng, J. & Ramamoorthy, A. (2021). Biophysical processes underlying cross-seeding in amyloid aggregation and implications in amyloid pathology. Biophys Chem 269, 106507.
[93] Subedi, S., Sasidharan, S., Nag, N., Saudagar, P. & Tripathi, T. (2022). Amyloid Cross-Seeding: Mechanism, Implication, and Inhibition. Molecules 27, 1776.
[94] Fox, T., Hunt, B. J., Ariëns, R. A. S., Towers, G. J., Lever, R., Garner, P. & Kuehn, R. (2023). Plasmapheresis to remove amyloid fibrin(ogen) particles for treating the post-COVID-19 condition. Cochrane Database of Systematic Reviews CD015775.
[95] Jaeger, B. R., Arron, H. E., Booyens, R. M., Kappert, C., van Helden, J., Weimer, M., Weingärtner, O., Seibel, R., Reichl, F., Khan, A. & Seidel, D. (2023). Long Covid Patients Successfully Treated by Means of Heparin-Mediated Extracorporeal LDL Precipitation (H.E.L.P.) Apheresis. Infect Dis Diag Treat 7, 216.
[96] Nunes, J. M., Kruger, A., Proal, A., Kell, D. B. & Pretorius, E. (2022). The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel) 15, 931.
[97] Nunes, J. M., Kell, D. B. & Pretorius, E. (2023). Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses. Blood Rev 60, 101075.
[98] Bradford Hill, A. (1965). Environment and Disease: Association or Causation? Proc R Soc Med 58, 295-300.
[99] Grixti, J. M., Theron, C. W., Salcedo-Sora, J. E., Pretorius, E. & Kell, D. B. (2024). Automated microscopic measurement of fibrinaloid microclots and their degradation by nattokinase, the main natto protease. bioRxiv, 2024.04.06.588397.
[100] Thagard, P. (1998). Explaining disease: Correlations, causes, and mechanisms. Minds and Machines 8, 61-78.
[101] Thagard, P. (1999). How scientists explain disease. Princeton University Press, Princeton, NJ.
[102] Thagard, P. (2007). Coherence, truth, and the development of scientific knowledge. Philosophy of Science 74, 28-47.
[103] Thagard, P. (2008). Explanatory Coherence. Reasoning: Studies of Human Inference and Its Foundations, 471-513.
[104] Thagard, P. (1998). Ulcers and Bacteria I: Discovery and Acceptance. Studies in History and Philosophy of Science. Part C 29, 107-36.
[105] Thagard, P. (1998). Ulcers and Bacteria II: Instruments, Experiments, and Social Interactions. Studies in History and Philosophy of Science. Part C 29, 107-36.
[106] Kell, D. B. & Welch, G. R. (2018). Belief: the baggage behind our being. OSF preprints, pnxcs https://osf.io/pnxcs/
[107] Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B. & Pretorius, E. (2020). Erythrocyte, platelet, serum ferritin and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int J Mol Sci 21, 8234.



Follow Prof Kell!