Introduction
The journal Research Practice in Thrombosis and Haemostasis recently chose to publish an opinion piece [1] under the title “Challenging the current hypothesis that thrombosis is responsible for the post-COVID-19 condition”, focusing on the theory (and in our cases the experimental measurement) of fibrinaloid microclots in the plasma of patients with Long COVID and related diseases. We are not entirely sure of the authors’ agenda (some of them have published similar diatribes before), and there seems to be something of an obsession with ‘Altmetric scores’ as if these had something to do with scientific truth, but the abstract contains the claims “We describe the development of this theory, examine the findings of a Cochrane review that critically appraises the “microclot” beliefs, and critically appraise the influential study relating clotting biomarkers to cognitive deficits. We conclude the inferences for the hypothesis are not based on evidence…”.
Unfortunately this article is so full of errors of fact and interpretation, as well as substantial omissions of the relevant literature, that we feel it necessary – as scientists who in contrast to the authors of [1] have actually worked on the experimental measurement of fibrinaloid microclots in these diseases – to help readers understand the true facts in an unbiased way. A variant of this rebuttal will be submitted under the authorship of Douglas Kell, Asad Khan and Resia Pretorius, but to avoid the inevitable delays caused by academic publishing timescales I am mounting the relevant arguments on this blog.
Frankly I do not really understand the motives of the people who are continuing to publish these completely ill-founded attacks on our work rather than doing some actual experiments themselves (?not invented here?). However, I consider them entirely reprehensible for reasons set out below, and will continue to call them out for as long as they choose to behave in this way. We already did so about the crazy Cochrane review that they managed to get published http://dbkgroup.org/dealing-with-clots/. Another rebuttal to an equally non-factual piece (full of claims that various papers say X when they actually say nothing or the opposite) is at [2]. While disinformation is certainly an unwelcome accompaniment to modern right-wing politics, it has no place in science, where the normal means of settling scientific debates is based on logic and the strength of arguments. Readers can decide who has them.
The authors of [1] first claim that “We initially critically appraised the research studies that had led to demand for apheresis treatment” (citing a Cochrane review [3]). We note that several of the authors of that paper, and one of its reviewers (Carson) are authors of [1], while Garner is actually an Editor of Cochrane. That particular Cochrane review attacked the microclots hypothesis and work of Pretorius and Kell, but was so extraordinarily biased and off target (e.g. entirely confusing plasmapheresis with HELP apheresis, on which none of us has ever published) that we wrote a full rebuttal. Cochrane has thus far pathetically refused even to append an online comment, but the rebuttal can be found at http://dbkgroup.org/dealing-with-clots/. Repeating erroneous facts when the authors know them to have been rebutted is very poor practice. As part of that review [3], it is mentioned by Hunt et al. [1] that microclots ‘were not unique’ to the PASC condition. The asininity of this ‘reasoning’ is made obvious simply by rehearsing the point that neither is the presence of glucose ‘unique’ to type 2 diabetes nor the presence of phenylalanine to phenylketonuria; what differentiates these diseases from a healthy state is the quantitative values of their levels – just as is the case with the microclots. We would assume that most readers would understand this rather elementary point, but seemingly it is necessary to go through it as this ‘non-uniqueness’ continues to be set down as if it is a serious argument (which obviously it is not).
Hunt et al also comment [1], given that the risk of deep vein thrombosis and pulmonary embolism in COVID-19 is much higher than the baseline risk, and that thromboembolic cardiovascular risk remains raised for at least a year in people recovering from severe acute COVID-19, that “these findings sparked the hypothesis that the persistence of these prothrombotic changes in acute COVID-19 may underlie the symptoms seen in patients with {PASC}”. Needless to say, no reference is given to seek to underpin their view as to what supposedly ‘sparked’ this hypothesis in someone’s mind, but elementary considerations show that (while both originate from SARS-CoV-2 and its proteins) it is not a defensible line of reasoning: (i) the demographics of the incidence in terms of age, BMI and gender are completely different for acute and long COVID, and (ii) the PHOSP study [4], that they also seek to criticise, actually uses acute COVID sufferers as controls for people who do not get PASC. Whatever logic is being used here is evidently not of the conventional variety. No ‘hypothesis’ was in fact necessary (it rarely is [5]), as we simply did the measurements.
They then claim [1] to “describe the development of the {microclot} theory”, in a manner that of course bears no relation to the true version, and given that they have made no contribution to it might be seen as rather arrogant. Readers might instead imagine that those of us who actually did develop the theory (which was based on extensive experimental data that Hunt et al. [1] almost completely omit to cite) might be best placed to discuss its development accurately, so we here do so. Another link is at http://dbkgroup.org/longcovid/. The microclot theory is airily described as ‘arising from a research team in South Africa’ when in fact the joint program led by Pretorius and Kell (who has been based in the UK throughout, presently at Liverpool and in the top 25 in the UK https://research.com/u/douglas-b-kell) is of over 10 years duration, and has produced more than 60 joint peer-reviewed publications.
The true history of microclot theory and measurements
Pretorius and colleagues had long noticed that blood could clot into a highly anomalous form, using measurements in the electron microscope (e.g. [6; 7]), including (with Kell, based in part on [8; 9]) the effects of unliganded iron [10; 11]. At this time these anomalous clots were referred to as ‘dense matted deposits’. The discovery that they were actually amyloid in nature, and could be induced by tiny amounts of bacterial cell wall material [12; 13] led to the ability to make measurements in a far more high-throughput manner using fluorogenic stains such as thioflavin T [14] and the AmytrackerTM [13; 15; 16] dyes. We also observed the microclots in a large number of chronic inflammatory diseases such as Alzheimer’s [15; 17-19], Parkinson’s [15; 20; 21], type 2 diabetes [15-17; 22] and rheumatoid artritis (e.g. [23]), each of which has an infectious origin [24]. The fact that these clots were amyloid in nature explained striaghtforwardly their resistance to the fibrinolysis that would normally remove them [14; 25; 26].
It was obvious from the earliest times that acute COVID involves coagulopathies, and we discovered the existence of extensive microclots that stained with amyloid stains in the plasma of sufferers from acute COVID [22; 27-29]. Measurements of microclots in sufferers from Long COVID (post-acute sequelae of CoOVID or PASC) also showed a far higher prevalence of microclots in their plasma than was observed in that of the controls [30-33], although the later arrival of vaccines containing or inducing spike protein added significant nuance to the distinction [34]. In particular, we showed that the spike protein alone (as with bacterial cell wall components) was able to induce the amyloidogenic microclots in normal plasma [35], and that the extent of these fibrinaloid microclots was correlated with the virulence of the variant [36]; this latter observation provides an important control or indication that the microclots are actually on the disease pathway. The amyloidogenic nature of the spike protein is itself also well established [37].
Similar studies also point up the role of fibrinaloid microclots and coagulopathies in ME/CFS [38-40], a condition showing many similarities to PASC [41].
Note too that the roles of fibrin amyloid microclots in Long COVID [34; 42; 43](see also [44]), and in disseminated intravascular coagulation (OR = 51) and 28-day mortality in intensive care patients (OR = 5.4) [45] have been determined in laboratories entirely independent of those of Pretorius and Kell. Various studies have also shown clearly the existence of fibrin inside the clots (along with many other proteins [30; 45; 46]), so by all appropriate usages they are correctly referred to as (micro)clots. Bizarrely, despite this array of papers supporting the existence of microclots in Long COVID and other diseases, along with the causative and interventional evidence such as adding spike protein to normal plasma, Hunt et al. [1] cite only one [30]. For them the other papers are seemingly ‘unknown knowns’; as phrased by Mike Hann [47] “these are those things that are known but have become unknown, either because we have never learnt them, or forgotten about them, or more dangerously chosen to ignore.”
Coherence of theory, evidence and mechanism
In addition to the hypothesis and evidence, the microclots theory, which follows the principle of scientific coherence [48-50], also provides a mechanistic basis for the otherwise multifarious symptomes of Long COVID [51], for the development of post-exertional malaise [52], for the induction of autoantibodies [26], for the development of postural orthostatic tachycardia syndrome (POTS) [53] and even for atrial fibrillation [54]. Having a clear (and testable) mechanism is an important part of a scientific explanation.
So while the initial discovery of amyloid-containing microclots [12] was broadly hypothesis-based (see [5]) (around the role of infection in chronic, inflammatory diseases, the ‘microclot theory’ is thoroughly grounded in very extensive experimental evidence, even if Hunt et al. [1] choose to ignore it. The risible claim [1] “we conclude {that} the inferences for the hypothesis are not based on evidence…” thus does not sit easily with the facts and extensive papers cited above – and facts that are, after all, very easily checkable by those who wish to. At least some of the authors of [1] should be qualified to judge them.
This said, a number of papers illustrating the value of anticoagulant therapy (leave along hundreds of grateful patients, who suffer dreadfully [55]) do not yet appear in the peer-reviewed scientific literature, but are available in preprint form (e.g. [56]), as is a study of the utility of fibrinolytic enzymes that are available as nutraceuticals [57].
Hunt et al. [1] also comment that endothelial dysfunction may also be involved in PASC. This is well established [58; 59], and as with the persistence of virus [60] that can induce continuing microclot production, not at all at odds with the microclot theory which, like anything else in biomedicine with ostensibly ‘competing’ theories (e.g. [61; 62]), needs to be seen more holistically within a systems biology framework (Figure 1 click on Figure for full size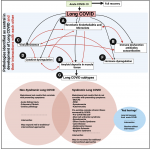 ).
).
Figure 1: An overview figure to show the major pathologies (black arrows) involved in the long COVID, as well as their interactions with each other (red arrows). A) Thrombotic endothelialitis and microclots as described and reviewed by [26; 30-32; 43; 46; 51; 52; 59; 63-65]; B)Immune dysfunction and autoantibodies [26; 32; 66; 67]; C) Viral persistence [41; 52; 60]; D) Cytokine dysregulation [59; 67; 68]; E) Muscle involvement [42]; F) hormone dysregulation [68]; G) subtypes of Long COVID as a result of the key pathologies driving the symptoms [32; 69; 70].
Given the extensive evidence supporting the microclot theory, what is actually needed is a proper Randomised Controlled Trial of the correct therapies aimed at anticoagulation and platelet inhibition. Such trials would necessarily include monitoring of bleeding potential using the correct methods such as thromboelastography (TEG), as we have consistently advocated (e.g. [28; 36]). The performance of these trials would be made much easier if efforts to discredit our well-established and coherent bodies of evidence by pretending they do not exist, seemingly in an attempt to disrupt patient care (which would amount to medical malpractice if carried out by clinicians), were to cease.
Ignorance of evidence may or may not be evidence of ignorance, but there is no excuse for it among those who wish to be taken seriously.
References
[1] Hunt, B. J., Kuehn, R., Fox, T., Carson, A., Scandrett, K., Davey Smith, G. & Garner, P. (2024). Challenging the current hypothesis that thrombosis is responsible for the post-COVID-19 condition. Res Pract Thromb Haemost 8, e102442
[2] Kell, D. B., Khan, M. A., Laubscher, G. J. & Pretorius, E. (2024). Uncertainties about the roles of anticoagulation and microclots in postacute sequelae of SARS-CoV-2 infection: comment from Kell et al. J Thromb Haemost 22, 565-568.
[3] Fox, T., Hunt, B. J., Ariëns, R. A. S., Towers, G. J., Lever, R., Garner, P. & Kuehn, R. (2023). Plasmapheresis to remove amyloid fibrin(ogen) particles for treating the post-COVID-19 condition. Cochrane Database of Systematic Reviews CD015775.
[4] Taquet, M., Skorniewska, Z., Hampshire, A., Chalmers, J. D., Ho, L. P., Horsley, A., Marks, M., Poinasamy, K., Raman, B., Leavy, O. C., Richardson, M., Elneima, O., McAuley, H. J. C., Shikotra, A., Singapuri, A., Sereno, M., Saunders, R. M., Harris, V. C., Houchen-Wolloff, L., Greening, N. J., Mansoori, P., Harrison, E. M., Docherty, A. B., Lone, N. I., Quint, J., Sattar, N., Brightling, C. E., Wain, L. V., Evans, R. E., Geddes, J. R., Harrison, P. J. & Phosp-Covid Study Collaborative Group. (2023). Acute blood biomarker profiles predict cognitive deficits 6 and 12 months after COVID-19 hospitalization. Nat Med 29, 2498-2508.
[5] Kell, D. B. & Oliver, S. G. (2004). Here is the evidence, now what is the hypothesis? The complementary roles of inductive and hypothesis-driven science in the post-genomic era. Bioessays 26, 99-105.
[6] Pretorius, E., Swanepoel, A. C., Oberholzer, H. M., van der Spuy, W. J., Duim, W. & Wessels, P. F. (2011). A descriptive investigation of the ultrastructure of fibrin networks in thrombo-embolic ischemic stroke. J Thromb Thrombolysis 31, 507-13.
[7] Pretorius, E., Oberholzer, H. M., van der Spuy, W. J., Swanepoel, A. C. & Soma, P. (2011). Qualitative scanning electron microscopy analysis of fibrin networks and platelet abnormalities in diabetes. Blood Coagul Fibrinol 22, 463-7.
[8] Kell, D. B. (2009). Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom 2, 2
[9] Kell, D. B. (2010). Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol 577, 825-889. .
[10] Pretorius, E., Vermeulen, N., Bester, J., Lipinski, B. & Kell, D. B. (2013). A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: the use of scanning electron microscopy. Toxicol Mech Methods 23, 352-359.
[11] Pretorius, E., Bester, J., Vermeulen, N., Lipinski, B., Gericke, G. S. & Kell, D. B. (2014). Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents. PLoS One 9, e85271.
[12] Pretorius, E., Mbotwe, S., Bester, J., Robinson, C. J. & Kell, D. B. (2016). Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J R Soc Interface 123, 20160539.
[13] Pretorius, E., Page, M. J., Hendricks, L., Nkosi, N. B., Benson, S. R. & Kell, D. B. (2018). Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: assessment with novel Amytracker™ stains. J R Soc Interface 15, 20170941.
[14] Kell, D. B. & Pretorius, E. (2017). Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Progr Biophys Mol Biol 123, 16-41.
[15] de Waal, G. M., Engelbrecht, L., Davis, T., de Villiers, W. J. S., Kell, D. B. & Pretorius, E. (2018). Correlative Light-Electron Microscopy detects lipopolysaccharide and its association with fibrin fibres in Parkinson’s Disease, Alzheimer’s Disease and Type 2 Diabetes Mellitus. Sci Rep 8, 16798.
[16] Pretorius, E., Page, M. J., Engelbrecht, L., Ellis, G. C. & Kell, D. B. (2017). Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc Diabetol 16, 141.
[17] Page, M. J., Thomson, G. J. A., Nunes, J. M., Engelbrecht, A. M., Nell, T. A., de Villiers, W. J. S., de Beer, M. C., Engelbrecht, L., Kell, D. B. & Pretorius, E. (2019). Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Sci Rep 9, 3102.
[18] Pretorius, E., Bester, J. & Kell, D. B. (2016). A bacterial component to Alzheimer-type dementia seen via a systems biology approach that links iron dysregulation and inflammagen shedding to disease J Alzheimers Dis 53, 1237-1256.
[19] Pretorius, E., Bester, J., Page, M. J. & Kell, D. B. (2018). The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Frontiers Aging Neurosci 10, 257.
[20] Pretorius, E., Page, M. J., Mbotwe, S. & Kell, D. B. (2018). Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PlosOne 13, e0192121.
[21] van Vuuren, M. J., Nell, T. A., Carr, J. A., Kell, D. B. & Pretorius, E. (2021). Iron dysregulation and inflammagens related to oral and gut health are central to the development of Parkinson’s disease. Biomolecules 11, 30.
[22] Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. & Kell, D. B. (2020). Prevalence of readily detected amyloid blood clots in ‘unclotted’ Type 2 Diabetes Mellitus and COVID-19 plasma: A preliminary report. Cardiovasc Diabetol 19, 193.
[23] Pretorius, E., Akeredolu, O.-O., Soma, P. & Kell, D. B. (2017). Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp Biol Med 242, 355-373.
[24] Kell, D. B. & Pretorius, E. (2018). No effects without causes. The Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev 93, 1518-1557.
[25] Kell, D. B. & Pretorius, E. (2015). The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen). Integr Biol 7, 24-52.
[26] Kell, D. B. & Pretorius, E. (2023). Are fibrinaloid microclots a cause of autoimmunity in Long Covid and other post-infection diseases? Biochem J 480, 1217-1240.
[27] Grobler, C., Maphumulo, S. C., Grobbelaar, L. M., Bredenkamp`, J., Laubscher, J., Lourens, P. J., Steenkamp, J., Kell, D. B. & Pretorius, E. (2020). COVID-19: The Rollercoaster of Fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes. Int J Mol Sci 21, 5168.
[28] Laubscher, G. J., Lourens, P. J., Venter, C., Kell, D. B. & Pretorius, E. (2021). TEG®, Microclot and Platelet Mapping for Guiding Early Management of Severe COVID-19 Coagulopathy. J Clin Med 10, 5381.
[29] Pretorius, E., Venter, C., Laubscher, G. J., Lourens, P. J., Steenkamp, J. & Kell, D. B. (2020). Prevalence of amyloid blood clots in COVID-19 plasma. medRxiv, 2020.07.28.20163543v1.
[30] Pretorius, E., Vlok, M., Venter, C., Bezuidenhout, J. A., Laubscher, G. J., Steenkamp, J. & Kell, D. B. (2021). Persistent clotting protein pathology in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol 20, 172.
[31] Pretorius, E., Venter, C., Laubscher, G. J., Kotze, M. J., Oladejo, S., Watson, L. R., Rajaratnam, K., Watson, B. W. & Kell, D. B. (2022). Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc Diabetol 21, 148.
[32] Turner, S., Khan, M. A., Putrino, D., Woodcock, A., Kell, D. B. & Pretorius, E. (2023). Long COVID: pathophysiological factors and abnormal coagulation. Trends Endocrinol Metab 34, 321-344.
[33] Turner, S., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2023). Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model Heliyon 9, e19605.
[34] Dalton, C. F., de Oliveira, M. I. R., Stafford, P., Peake, N., Kane, B., Higham, A., Singh, D., Jackson, N., Davies, H., Price, D., Duncan, R., Tattersall, N., Barnes, A. & Smith, D. P. (2024). Increased fibrinaloid microclot counts in platelet-poor plasma are associated with Long COVID. medRxiv, 2024.04.04.24305318.
[35] Grobbelaar, L. M., Venter, C., Vlok, M., Ngoepe, M., Laubscher, G. J., Lourens, P. J., Steenkamp, J., Kell, D. B. & Pretorius, E. (2021). SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci Rep 41, BSR20210611.
[36] Grobbelaar, L. M., Kruger, A., Venter, C., Burger, E. M., Laubscher, G. J., Maponga, T. G., Kotze, M. J., Kwaan, H. C., Miller, J. B., Fulkerson, D., Huff, W., Chang, E., Wiarda, G., Bunch, C. M., Walsh, M. M., Raza, S., Zamlut, M., Moore, H. B., Moore, E. E., Neal, M. D., Kell, D. B. & Pretorius, E. (2022). Relative hypercoagulopathy of the SARS-CoV-2 Beta and Delta variants when compared to the less severe Omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin Thromb Haemost 48, 858-868.
[37] Nyström, S. & Hammarström, P. (2022). Amyloidogenesis of SARS-CoV-2 Spike Protein. J Am Chem Soc 144, 8945-8950.
[38] Nunes, J. M., Kruger, A., Proal, A., Kell, D. B. & Pretorius, E. (2022). The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals (Basel) 15, 931.
[39] Nunes, J. M., Kell, D. B. & Pretorius, E. (2023). Cardiovascular and haematological pathology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): a role for Viruses. Blood Rev 60, 101075.
[40] Nunes, J. M., Vlok, M., Proal, A., Kell, D. B. & Pretorius, E. (2024). Data-independent LC-MS/MS analysis of ME/CFS plasma reveals a dysregulated coagulation system, endothelial dysfunction, downregulation of complement machinery. Cardiovasc. Diabetol., in press.
[41] Proal, A. D. & VanElzakker, M. B. (2021). Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front Microbiol 12, 698169.
[42] Appelman, B., Charlton, B. T., Goulding, R. P., Kerkhoff, T. J., Breedveld, E. A., Noort, W., Offringa, C., Bloemers, F. W., van Weeghel, M., Schomakers, B. V., Coelho, P., Posthuma, J. J., Aronica, E., Joost Wiersinga, W., van Vugt, M. & Wüst, R. C. I. (2024). Muscle abnormalities worsen after post-exertional malaise in long COVID. Nat Commun 15, 17.
[43] Okuducu, Y. K., Boribong, B., Ellett, F., Hajizadeh, S., VanElzakker, M., Haas, W., Pillai, S., Fasano, A., Irimia, D. & Yonker, L. (2024). Evidence Circulating Microclots and Activated Platelets Contribute to Hyperinflammation Within Pediatric Post Acute Sequala of COVID. Am J Respir Crit Care Med 209, A2247.
[44] Di Gennaro, L., Valentini, P., Sorrentino, S., Ferretti, M. A., De Candia, E., Basso, M., Lancellotti, S., De Cristofaro, R., De Rose, C., Mariani, F., Morello, R., Lazzareschi, I., Sigfrid, L., Munblit, D. & Buonsenso, D. (2022). Extended coagulation profile of children with Long Covid: a prospective study. Sci Rep 12, 18392.
[45] Schofield, J., Abrams, S. T., Jenkins, R., Lane, S., Wang, G. & Toh, C. H. (2024). Amyloid-fibrinogen aggregates (“microclots”) predict risks of Disseminated Intravascular Coagulation and mortality. Blood Adv 8, 2499-2508.
[46] Kruger, A., Vlok, M., Turner, S., Venter, C., Laubscher, G. J., Kell, D. B. & Pretorius, E. (2022). Proteomics of fibrin amyloid microclots in Long COVID/ Post-Acute Sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc Diabetol 21, 190.
[47] Hann, M. M. (2011). Molecular obesity, potency and other addictions in drug discovery. MedChemComm 2, 349-355.
[48] Thagard, P. (1999). How scientists explain disease. Princeton University Press, Princeton, NJ.
[49] Thagard, P. (2007). Coherence, truth, and the development of scientific knowledge. Philosophy of Science 74, 28-47.
[50] Thagard, P. (2008). Explanatory Coherence. Reasoning: Studies of Human Inference and Its Foundations, 471-513.
[51] Kell, D. B., Laubscher, G. J. & Pretorius, E. (2022). A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem J 479, 537-559.
[52] Kell, D. B. & Pretorius, E. (2022). The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: evidence, mechanisms, and therapeutic implications. Biochem J 479, 1653-1708.
[53] Kell, D. B., Khan, M. A., Kane, B., Lip, G. Y. H. & Pretorius, E. (2024). Possible role of fibrinaloid microclots in Postural Orthostatic Tachycardia Syndrome (POTS): focus on Long COVID. J Personalised Medicine 14, 170.
[54] Kell, D. B., Lip, G. Y. H. & Pretorius, E. (2024). Fibrinaloid Microclots and Atrial Fibrillation. Biomedicines 12, 891.
[55] Sawano, M., Wu, Y., Shah, R. M., Zhou, T., Arun, A. S., Khosla, P., Kaleem, S., Vashist, A., Bhattacharjee, B., Ding, Q., Lu, Y., Caraballo, C., Warner, F., Huang, C., Herrin, J., Putrino, D., Michelsen, T., Fisher, L., Adinig, C., Iwasaki, A. & Krumholz, H. M. (2024). Long COVID Characteristics and Experience: A Descriptive Study from the Yale LISTEN Research Cohort. Am J Med.
[56] Laubscher, G. J., Khan, M. A., Venter, C., Pretorius, J. H., Kell, D. B. & Pretorius, E. (2023). Treatment of Long COVID symptoms with triple anticoagulant therapy. https://www.researchsquare.com/article/rs-2697680/v1.
[57] Grixti, J. M., Theron, C. W., Salcedo-Sora, J. E., Pretorius, E. & Kell, D. B. (2024). Automated microscopic measurement of fibrinaloid microclots and their degradation by nattokinase, the main natto protease. bioRxiv, 2024.04.06.588397.
[58] Turner, S., Naidoo, C., Usher, T., Kruger, A., Venter, C., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2022). Increased levels of inflammatory molecules in blood of Long COVID patients point to thrombotic endotheliitis. medRxiv, 2022.10.13.22281055.
[59] Turner, S., Naidoo, C. A., Usher, T. J., Kruger, A., Venter, C., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2024). Increased Levels of Inflammatory and Endothelial Biomarkers in Blood of Long COVID Patients Point to Thrombotic Endothelialitis. Semin Thromb Hemost 50, 288-294.
[60] Proal, A. D., VanElzakker, M. B., Aleman, S., Bach, K., Boribong, B. P., Buggert, M., Cherry, S., Chertow, D. S., Davies, H. E., Dupont, C. L., Deeks, S. G., Eimer, W., Ely, E. W., Fasano, A., Freire, M., Geng, L. N., Griffin, D. E., Henrich, T. J., Iwasaki, A., Izquierdo-Garcia, D., Locci, M., Mehandru, S., Painter, M. M., Peluso, M. J., Pretorius, E., Price, D. A., Putrino, D., Scheuermann, R. H., Tan, G. S., Tanzi, R. E., VanBrocklin, H. F., Yonker, L. M. & Wherry, E. J. (2023). SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). Nat Immunol 24, 1616-1627.
[61] Kell, D. B. & Kenny, L. C. (2016). A dormant microbial component in the development of pre-eclampsia. Front Med Obs Gynecol 3, 60.
[62] Grobler, C., van Tongeren, M., Gettemans, J., Kell, D. & Pretorius, E. (2023). Alzheimer-type dementia: a systems view provides a unifying explanation of its development. J Alz Dis 91, 43-70.
[63] Altmann, D. M., Whettlock, E. M., Liu, S., Arachchillage, D. J. & Boyton, R. J. (2023). The immunology of long COVID. Nat Rev Immunol.
[64] Kell, D. B., Khan, M. A., Kane, B., Lip, G. Y. H. & Pretorius, E. (2024). Possible Role of Fibrinaloid Microclots in Postural Orthostatic Tachycardia Syndrome (POTS): Focus on Long COVID. J Pers Med 14, 170.
[65] Turner, S., Laubscher, G. J., Khan, M. A., Kell, D. B. & Pretorius, E. (2023). Rapid flow cytometric analysis of fibrin amyloid microclots in Long COVID. Preprint at https://www.researchsquare.com/article/rs-2731434/v1, submitted.
[66] Klein, J., Wood, J., Jaycox, J. R., Dhodapkar, R. M., Lu, P., Gehlhausen, J. R., Tabachnikova, A., Greene, K., Tabacof, L., Malik, A. A., Silva Monteiro, V., Silva, J., Kamath, K., Zhang, M., Dhal, A., Ott, I. M., Valle, G., Pena-Hernandez, M., Mao, T., Bhattacharjee, B., Takahashi, T., Lucas, C., Song, E., McCarthy, D., Breyman, E., Tosto-Mancuso, J., Dai, Y., Perotti, E., Akduman, K., Tzeng, T. J., Xu, L., Geraghty, A. C., Monje, M., Yildirim, I., Shon, J., Medzhitov, R., Lutchmansingh, D., Possick, J. D., Kaminski, N., Omer, S. B., Krumholz, H. M., Guan, L., Dela Cruz, C. S., van Dijk, D., Ring, A. M., Putrino, D. & Iwasaki, A. (2023). Distinguishing features of long COVID identified through immune profiling. Nature 623, 139-148.
[67] Saito, S., Shahbaz, S., Osman, M., Redmond, D., Bozorgmehr, N., Rosychuk, R. J., Lam, G., Sligl, W., Cohen Tervaert, J. W. & Elahi, S. (2024). Diverse immunological dysregulation, chronic inflammation, and impaired erythropoiesis in long COVID patients with chronic fatigue syndrome. J Autoimmun 147, 103267.
[68] Silva, J., Takahashi, T., Wood, J., Lu, P., Tabachnikova, A., Gehlhausen, J. R., Greene, K., Bhattacharjee, B., Monteiro, V. S., Lucas, C., Dhodapkar, R. M., Tabacof, L., Pena-Hernandez, M., Kamath, K., Mao, T., McCarthy, D., Medzhitov, R., van Dijk, D., Krumholz, H. M., Guan, L., Putrino, D. & Iwasaki, A. (2024). Sex differences in symptomatology and immune profiles of Long COVID. medRxiv.
[69] Al-Aly, Z., Xie, Y. & Bowe, B. (2021). High-dimensional characterization of post-acute sequelae of COVID-19. Nature 594, 259-264.
[70] Al-Aly, Z. & Topol, E. (2024). Solving the puzzle of Long Covid. Science 383, 830-832.



Follow Prof Kell!